 |
 |
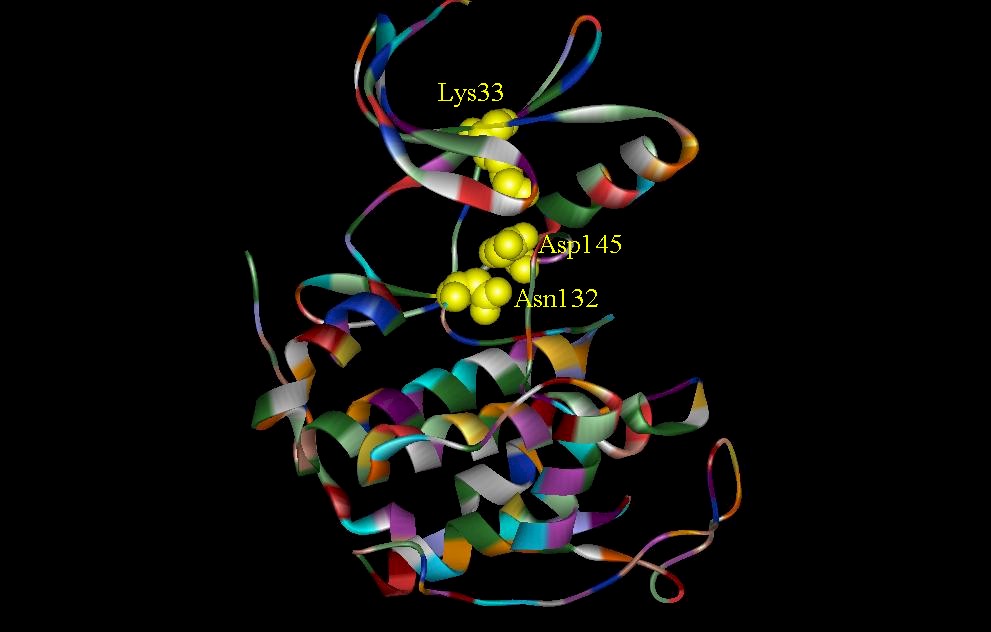
The structure of CDK2 is determined by MIR method and CDK-Mg2+ ATP complex by Fourier analysis (DeBondt et. al., 1993). CDK2 can be divided into two lobes. The CDK2-Mg2+ ATP binary complex indicates that ATP interacts with several residues (Lys33 and Asp145) between the two lobes (figure 2). The ATP phosphate is held in place by these residues. The C subunit of the cAMP-dependent protein kinase is also divided into two lobes. The smaller lobe is associated with MgATP binding and the larger lobe is associated with peptide recognition. Figure 3 shows a comparison between the crucial residues involved in ATP-binding site interactions seen in CDK2 and the C subunit of cAMP-dependent protein kinase. Two critical residues, Lys33 and Asp145 are involved in ATP phosphate binding in CDK2 and the equivalent residues in the C subunit are Lys72 and Asp184. Asn132 of CDK2 is important because it is involved with the co-ordination of Mg2+. Even though this is not shown in the drawings, it is important to note that Lys33 and Asn132 have different orientations in the presence and absence of ATP (DeDeBondt, et. al., 1993).


Figure 3. Catalytic sites in CDK2 and catalytic subunit of cAMP. (A) Structure shows three residues (Lys33, Asp145, and Asn132) involved in the CDK2-Mg2+ ATP complex. ATP phosphates are anchored by ironic and hydrogen bonding interactions with Lys33 and Asp145. Asn132 in addition to Lys33 and Asp145 is involved in the co-ordination of Mg2+. Not shown is a water molecule which is also involved in the co-ordination of Mg2+. (B) Two critical residues, Lys72 and Asp184, which are involved in phosphate orientation and metal ion coordination in the catalytic subunit of cAMP.
Two important loops present in each of the kinases are worthy of further examination. First, the glycine loop in CDK2 include inhibitory phosphorylation sites (Thr14 and Tyr15) that also serves as a phosphate anchor in ATP binding (DeDeBondt, et. al., 1993). The hydroxyl of Thr14 closely resemble the gamma-phosphate of ATP (DeDeBondt, et. al., 1993). Phosphorylation at this position will disrupt the conformation of ATP phosphate (DeDeBondt, et. al., 1993). The catalytic loop of CDK2 is involved with the ATP binding site interactions as mentioned earlier. The glycine-rich loop embedded in the small lobe of the C subunit serves as an anchor for the phosphates of MgATP (figure5) while the catalytic loop embedded in the large loop is essential for peptide binding and catalysis (Knighton, et. al., 1991). This catalytic loop is also important because it contains invariant amino acids (figure 6) that interact with the gamma-phosphate and help to facilitate catalysis (Zheng, et. al., 1992).
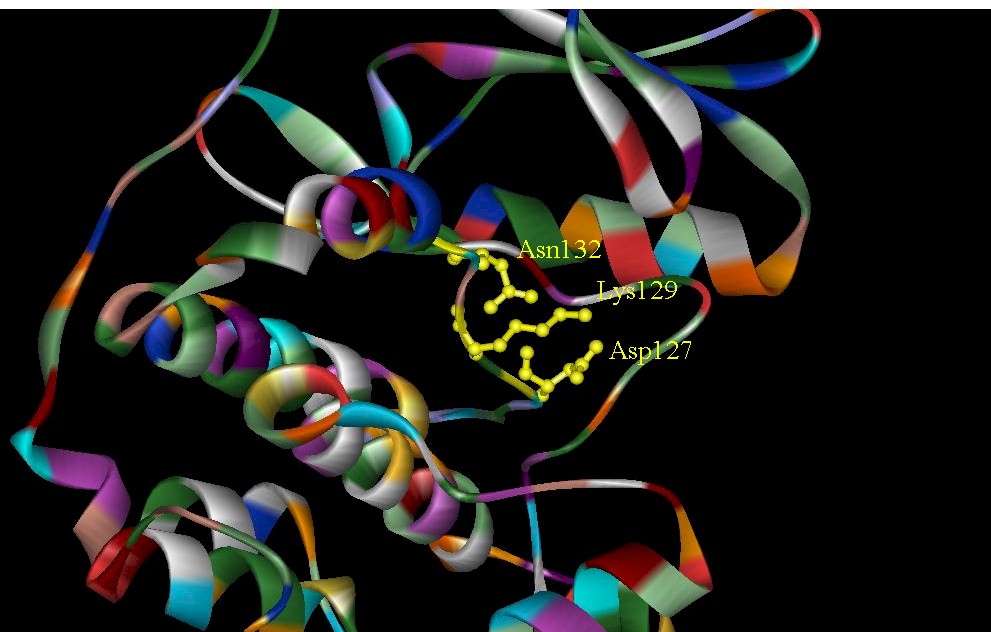
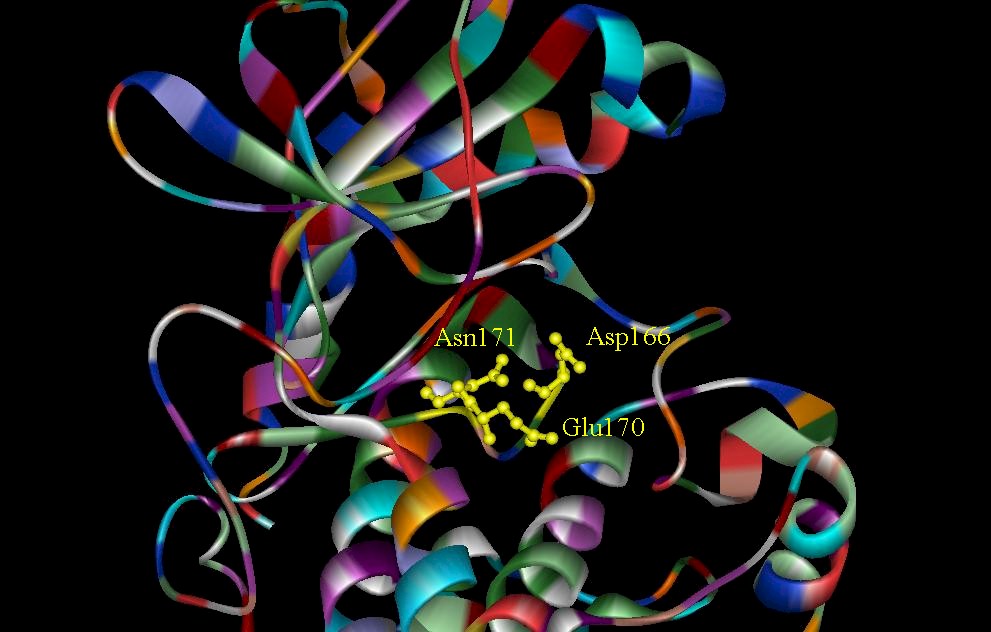
Figure 4. Close-up view of the catalytic loop in CDK2 and catalytic subunit of cAMP. (A) Conserved residues in CDK2 of the catalytic loop. These residues interact with the ATP-binding-site. (B) Residues found in the catalytic loop. The catalytic loop is essential for peptide binding and catalysis.

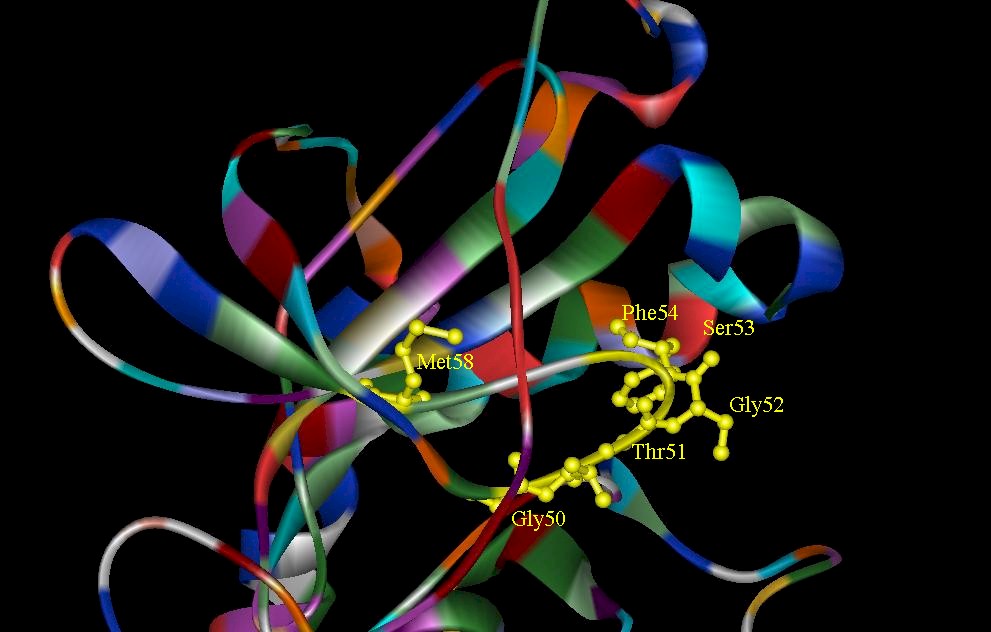
Figure 5. (A) A close-up view of residues Thr14 and Tyr15 in the glycine-rich loop in the CDK2. They serve as phosphate anchors in ATP binding. (B) The glycine-rich sequence of the catalytic subunit of cAMP is highlighted yellow. The glycine-rich loop serves as an anchor for the phosphate of MgATP.
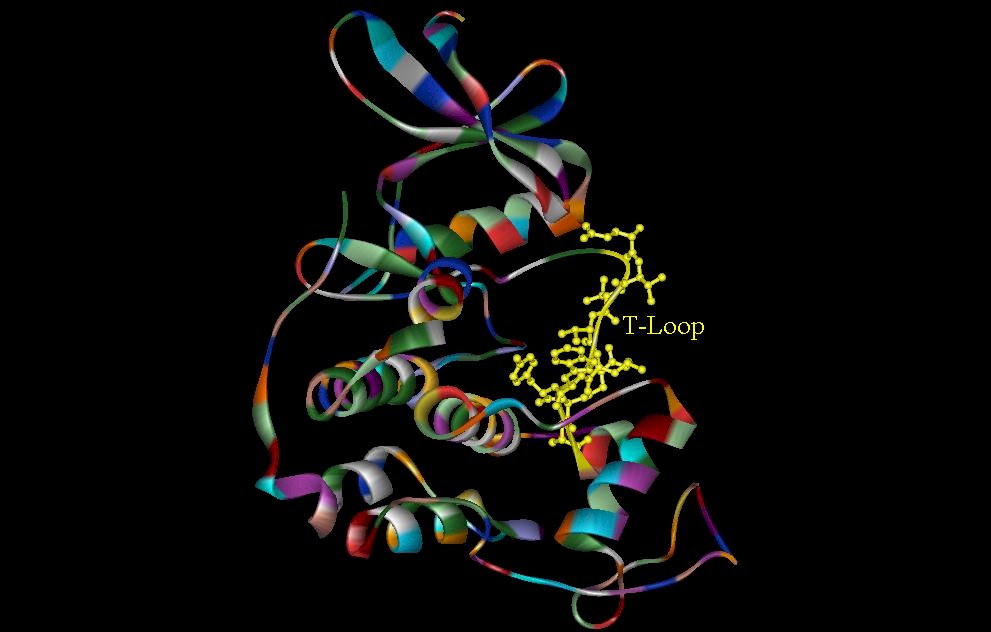
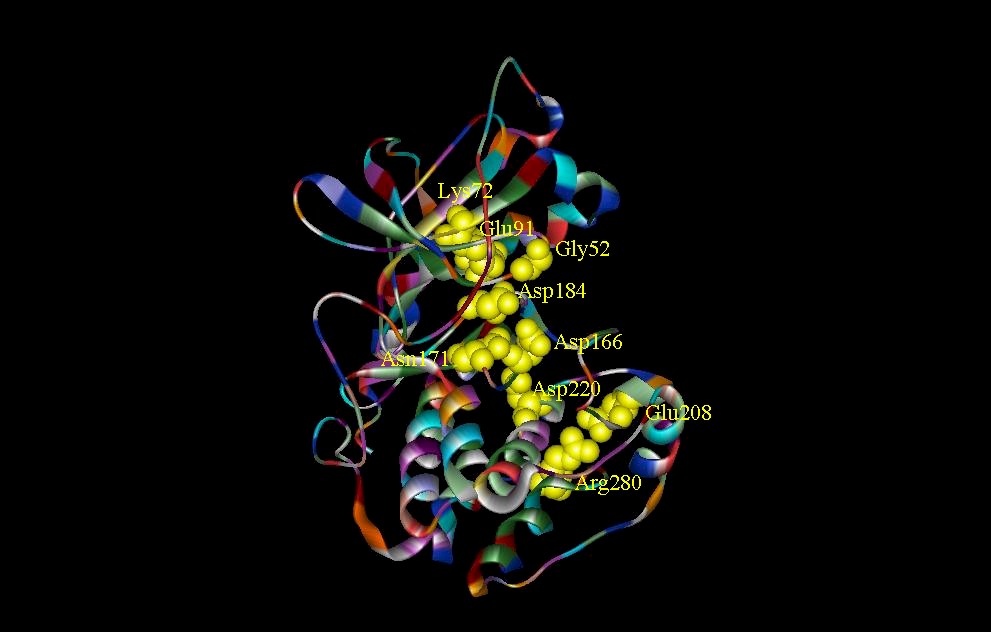
Figure 6. (A) The T-Loop (residues 152-170) of the CDK2. Several residues in this loop make the gamma-phosphate of ATP inaccessible, making effective binding of the protein substrate very unlikely. (B) The invariant amino acids that interact with the gamma-phosphate and help to facilitate catalysis (Zheng, et. al., 1992).
The inhibitor found in the C subunit of cAMP-dependent protein kinase is generated by the replacement of the Ser with an Ala (Knighton, et. al., 1991). The heat stable protein kinase inhibitor PKI (5-24) convey high affinity binding site interactions (figure 7). The reason for such high affinity binding of the PKI (5-24) is attributed to the hydrophobic interactions involving the Phe side chain (Knighton, et. al., 1991). Figure 7 shows the hydrophobic residues involved in these hydrophobic interactions. The replacement of Phe with Ala can cause a hundred fold increase in Ki (Knighton, et. al., 1991). The main inhibition in CDK2 is seen in the T-Loop and its interactions with ATP binding sites. Some residues in this region blocks accessibility to the gamma-phosphate of ATP, therefore the T-Loop seems to act as an auto-inhibitor of the protein substrate binding. Researchers hypothesize that the low kinase activity of CDK2 is attributed to the T-Loop (DeDeBondt, et. al., 1993).

Figure 7. High-affinity binding site interactions in the C subunit of cAMP-dependent protein kinase with the hydrophobic residues (235-239) highlighted in yellow..